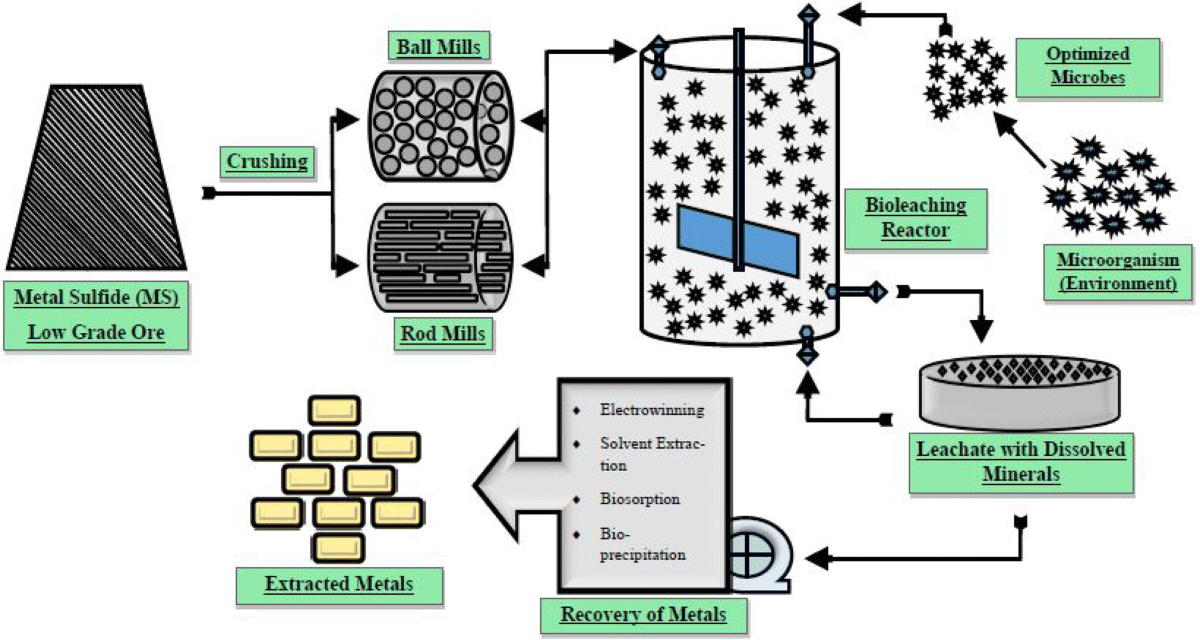
Bioleaching and hydrometallurgical techniques are advanced methods that enhance the extraction of metals from sulfide ores, particularly for ores that are difficult or uneconomical to process through traditional methods like smelting. Both techniques utilize chemical and biological processes to extract metals in a more efficient and environmentally friendly manner.
Here’s an overview of how they work and their benefits:
Bioleaching:
Bioleaching is a biological process where microorganisms, such as bacteria or fungi, are used to oxidize sulfide minerals and release the metals contained in them.
- Process Overview:
- Microorganisms such as Thiobacillus ferrooxidans and Acidithiobacillus thiooxidans are capable of oxidizing sulfide minerals (e.g., pyrite or chalcopyrite) into soluble forms like sulfates and metal ions.
- The bacteria oxidize the sulfide minerals in the ore, which releases sulfuric acid (H₂SO₄) and solubilizes metals such as copper, gold, nickel, and zinc into the solution.
- The process is often carried out in bioleach heaps, where crushed ore is stacked in piles and irrigated with acidic solutions that contain microorganisms. The bacteria break down the sulfide minerals over time, allowing the metals to dissolve.
- Advantages of Bioleaching:
- Lower Energy Requirements: Compared to traditional smelting, bioleaching requires significantly less energy, making it more cost-effective and environmentally friendly.
- Selective Extraction: Bioleaching can be highly selective for certain metals, particularly copper and gold, allowing for the efficient extraction of valuable metals from low-grade ores.
- Environmental Benefits: Bioleaching generates less pollution compared to conventional methods like roasting or smelting. It produces fewer greenhouse gases and avoids the need for high-temperature furnaces.
- Use of Low-Grade Ores: Bioleaching is especially useful for low-grade ores, which would otherwise be uneconomical to process using traditional methods. It makes it possible to extract metals from ores that are less rich in metal content.
- Challenges of Bioleaching:
- Slow Process: Bioleaching is relatively slow compared to traditional methods, which can be a limitation for large-scale operations.
- Microbial Control: The efficiency of bioleaching depends on maintaining the proper conditions for the microorganisms, such as pH, temperature, and oxygen levels.
- Environmental Risks: If not properly controlled, bioleaching can lead to the release of acidic effluents, which can harm the surrounding environment.
Hydrometallurgical Techniques:
Hydrometallurgy involves using aqueous (water-based) solutions to extract metals from ores, typically through processes like leaching, solvent extraction, and electrowinning. In the context of sulfide ores, hydrometallurgy can help extract metals by using chemical agents to dissolve metal sulfides and selectively separate valuable metals.
- Leaching:
- In hydrometallurgy, leaching is the process where an acidic or alkaline solution is used to dissolve valuable metals from sulfide ores.
- For sulfide ores, an acid (e.g., sulfuric acid) is often used to dissolve the sulfide minerals and release the metal ions into the solution.
- Pressure leaching and heap leaching are common methods used to improve the efficiency of the leaching process.
- For example, in copper extraction, copper sulfides (such as chalcopyrite) are treated with sulfuric acid, which dissolves copper into solution as copper sulfate.
- In hydrometallurgy, leaching is the process where an acidic or alkaline solution is used to dissolve valuable metals from sulfide ores.
- Solvent Extraction:
- After leaching, the metal-laden solution can undergo solvent extraction (SX), where an organic solvent selectively binds to the metal ions in solution, allowing them to be separated from other impurities.
- The solvent is then treated to separate the metal, which is usually done by electrowinning or precipitation.
- Electrowinning:
- Electrowinning involves applying an electric current to the metal-laden solution, causing the metal ions to be reduced and deposited onto a cathode as pure metal. This is commonly used to recover copper and other base metals.
- This process is widely used in the extraction of copper from heap leaching or tank leaching operations, where the copper solution is subjected to electrolysis to produce high-purity copper.
- Advantages of Hydrometallurgy:
- Lower Energy Consumption: Hydrometallurgy typically consumes less energy than traditional smelting, particularly for low-grade ores.
- Selective Metal Extraction: Hydrometallurgy allows for the selective extraction of valuable metals, which can be advantageous when dealing with ores containing multiple metals.
- Environmental Benefits: It generates fewer greenhouse gases and pollutants compared to high-temperature processing methods like smelting.
- Suitable for Complex Ores: Hydrometallurgical techniques can be used to treat complex ores that would be difficult to process with traditional methods.
- Challenges of Hydrometallurgy:
- Reagent Costs: Some hydrometallurgical processes require expensive chemicals or solvents, increasing operational costs.
- Contamination: The leaching solutions used in hydrometallurgy can sometimes become contaminated with impurities, requiring additional steps to purify the solution before further processing.
- Waste Management: Disposal of residual leach solutions, solvents, and tailings can pose environmental challenges, particularly if not properly treated.
How Bioleaching and Hydrometallurgical Techniques Enhance Metal Extraction from Sulfide Ores:
- Efficiency in Low-Grade Ores: Both bioleaching and hydrometallurgy enable the extraction of metals from low-grade sulfide ores, which might otherwise be uneconomical to process with conventional smelting methods.
- Environmental Benefits: These methods are generally more environmentally friendly than traditional processes, as they involve lower temperatures, reduced energy consumption, and fewer greenhouse gas emissions.
- Enhanced Recovery: Both techniques can be used in tandem to improve metal recovery. For instance, bioleaching can be used to pre-treat ores, making them more amenable to further hydrometallurgical processing (e.g., pressure leaching), which increases the overall recovery of valuable metals.
- Selective Extraction: Hydrometallurgy, especially with solvent extraction, allows for the selective recovery of individual metals from complex ores, which is crucial in ores with multiple metals (e.g., copper and gold).
Conclusion:
Both bioleaching and hydrometallurgical techniques enhance the extraction of metals from sulfide ores by providing more energy-efficient, selective, and environmentally friendly alternatives to traditional methods. While bioleaching is particularly useful for low-grade ores and offers low-cost extraction with minimal environmental impact, hydrometallurgical techniques are ideal for recovering metals with high precision, particularly when combined with advanced processes like solvent extraction and electrowinning. Both methods continue to evolve with advancements in technology, contributing to more sustainable mining practices.