Silicate minerals can significantly impact the effectiveness of flotation, leaching, and other separation processes in mineral processing due to their chemical and physical properties. Here’s how silicate minerals affect each of these methods:
1. Flotation
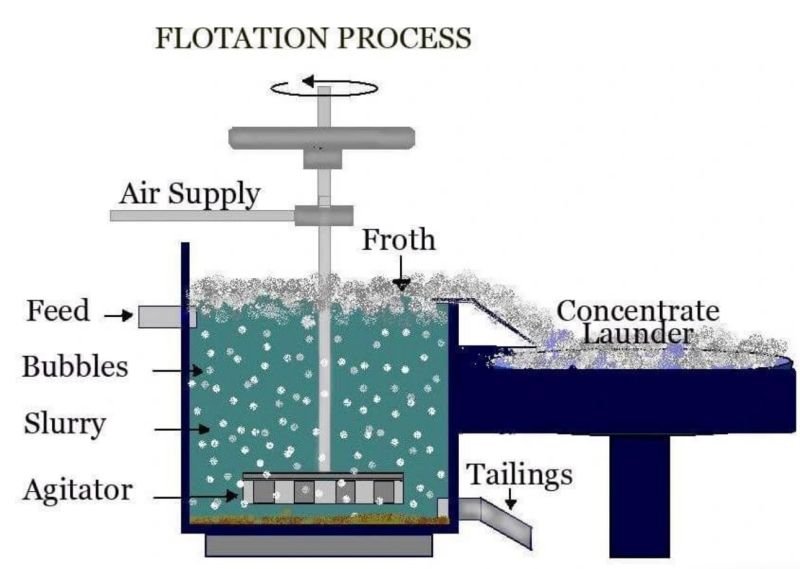
Flotation is a separation process that relies on differences in the surface chemistry of minerals to separate valuable minerals from gangue (waste material). Silicate minerals, particularly quartz, feldspar, and mica, present several challenges during flotation.
Impact of Silicates on Flotation:
- Low Flotation Response: Silicate minerals are often chemically inert and do not easily react with flotation reagents. As a result, they may not be readily separated from valuable minerals in ores.
- Example: Quartz, a common silicate mineral, is usually hydrophilic (water-attracting) and doesn’t form a stable froth in flotation processes. This makes it challenging to separate from hydrophobic (water-repellent) minerals like gold or copper.
- Interference with Reagents: Silicates can adsorb flotation reagents (collectors and frothers) that are meant to target valuable minerals, thus reducing the efficiency of the flotation process.
- Example: In feldspar flotation, silicate minerals such as quartz may interfere with the selectivity of collectors, leading to poor separation and lower recovery rates.
- Mixed Mineral Challenges: Silicate-rich ores that contain a variety of silicate minerals (e.g., feldspar, quartz, mica) make selective flotation difficult. Multiple reagents are often needed to target different minerals, complicating the process and increasing costs.
- Improvement: Modified flotation methods using specialized reagents (e.g., alkylammonium collectors) and controlling the pH can improve selectivity for silicates, but these methods can be more costly.
2. Leaching
Leaching is a process that uses chemicals (usually acids or alkalis) to dissolve metals from their ores, which is often used for silicate ores to extract metals like copper, gold, or nickel. However, silicate minerals can influence the efficiency of leaching in several ways.
Impact of Silicates on Leaching:
- Chemical Inertness: Many silicate minerals, such as quartz or feldspar, are chemically inert and do not react easily with typical leaching agents (e.g., cyanide, sulfuric acid). This means that silicate gangue does not contribute to metal extraction, which reduces the efficiency of the process.
- Example: In cyanide leaching for gold, silicate minerals like quartz do not contribute to gold recovery, but they still consume valuable reagents and may complicate the treatment.
- Increased Acid Consumption: Silicate minerals, particularly those rich in aluminum (like kaolinite) or magnesium (like serpentine), can consume large amounts of leaching acids. This increases the overall reagent consumption and makes the process less economical.
- Example: Bauxite (an aluminum silicate ore) requires significant amounts of caustic soda to dissolve the alumina, making the extraction process more energy- and resource-intensive.
- Silica Contamination: In leaching processes that involve cyanide or sulfuric acid, silicate gangue like quartz can contaminate the leachate, creating impurities in the final product and making purification steps more difficult.
- Example: Silica contamination in the leachate from gold ores can lead to unwanted reactions or adsorption of gold on the silica, reducing the efficiency of gold extraction.
3. Other Separation Processes
Gravity Separation
- Impact of Silicates: Silicate minerals can sometimes be separated by gravity methods if there is a significant difference in density between the silicate minerals and the target minerals. For example, garnet, a heavy silicate mineral, can be separated from lighter minerals like quartz using gravity-based methods (e.g., shaking tables or jigs). However, for minerals like quartz and feldspar, which are similar in density to the target minerals, gravity separation is less effective.
Magnetic Separation
- Impact of Silicates: Some silicate minerals, such as magnetite (Fe₃O₄), are magnetic and can be easily separated from non-magnetic silicates like quartz or feldspar. However, non-magnetic silicates (like quartz and mica) do not respond well to magnetic separation and can remain mixed with the valuable ore.
- Example: In iron ore beneficiation, magnetic separation is often used to separate magnetite (a magnetic silicate) from non-magnetic gangue silicates like quartz.
Hydrometallurgical Techniques
- Impact of Silicates: Silicate minerals can influence the efficiency of hydrometallurgical processes like solvent extraction and electrowinning. For example, the presence of silica in copper ores can complicate the process by causing scaling or clogging in the solvent extraction system, leading to lower efficiency in metal recovery.
4. Smelting and High-Temperature Processes
- Impact of Silicates on Smelting: In high-temperature smelting, silicate gangue often combines with other impurities to form slag, which must be removed to obtain the pure metal. Silicate minerals can increase the volume of slag and require additional fluxing agents (like lime) to facilitate slag formation.
- Example: In copper smelting, silicate gangue can lead to the formation of complex slags that need to be separated from the molten copper, requiring more energy and fluxing material.
Conclusion
Silicate minerals pose challenges in several mineral processing methods, including flotation, leaching, gravity separation, and smelting. Their chemical inertness, abrasiveness, and similar densities to valuable minerals can complicate separation and reduce recovery efficiency. Specialized techniques, such as the use of selective flotation reagents, acid treatment, and fluxing agents in smelting, are often required to address these challenges.