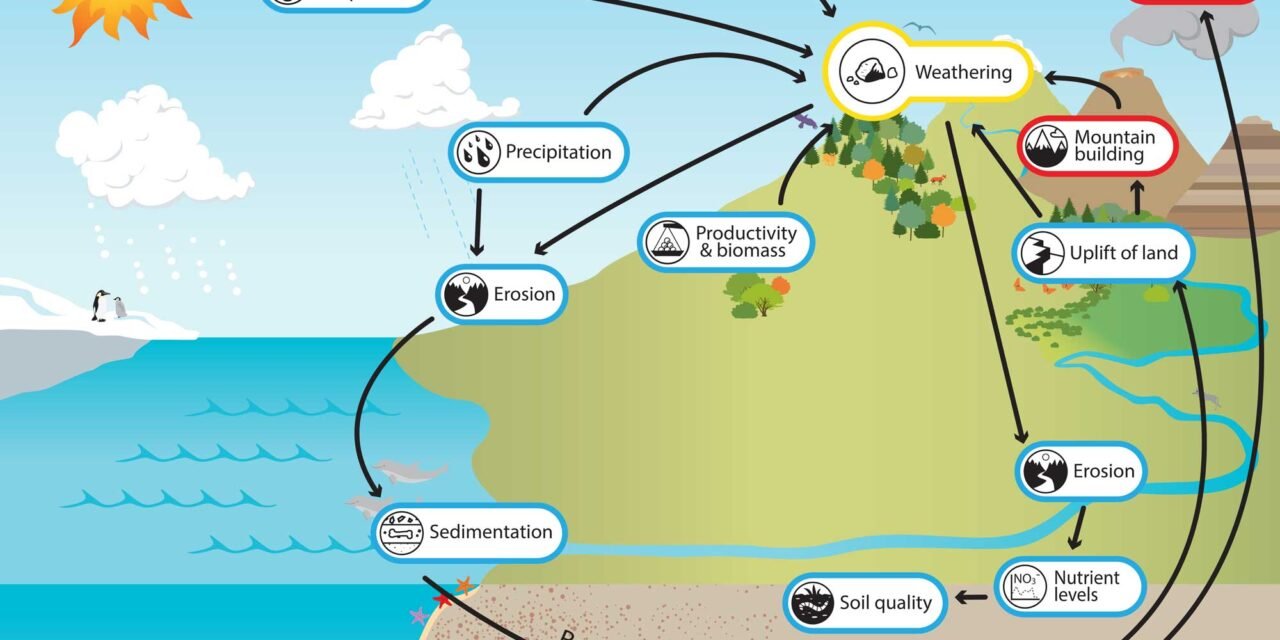
Weathering plays a crucial role in the formation of economically significant oxide mineral deposits by altering primary minerals and concentrating valuable metals in forms that are easier to extract. Weathering is the process by which rocks and minerals break down due to exposure to environmental conditions like rain, temperature fluctuations, and biological activity. Over time, this breakdown leads to the formation of secondary oxide minerals, which are often more stable and concentrated than their parent minerals. Here’s how weathering contributes to the formation of these important oxide deposits:
1. Breakdown of Primary Minerals
- Weathering of Sulfides and Silicates: Primary ore minerals such as sulfides (e.g., chalcopyrite or pyrite) and silicates (e.g., feldspar) are exposed to atmospheric oxygen and water. Over time, these minerals undergo oxidation and hydrolysis reactions, breaking down into secondary oxide minerals.
- Example: Iron sulfides (like pyrite) break down into iron oxides (such as hematite and goethite) during weathering, making iron available in a more easily processed oxide form.
- Copper sulfides (like chalcopyrite) can weather to form copper oxides (like malachite or azurite), which are more readily soluble in acidic solutions and easier to process.
2. Leaching and Concentration of Metals
- Leaching: As minerals weather, leaching occurs, in which water dissolves ions from minerals, especially metals like iron, aluminum, copper, and manganese. This process can concentrate metals in secondary oxide minerals, enriching the deposit and making it more valuable.
- Example: Bauxite (a major aluminum ore) forms from the weathering of aluminum-rich silicate rocks like feldspar. Over time, the weathering process removes silica and other impurities, leaving a concentrated deposit of gibbsite (Al(OH)₃), boehmite (AlO(OH)), and diaspore (AlO(OH)), which are valuable aluminum ores.
3. Formation of Lateritic Soils
- Laterite Formation: In tropical and subtropical regions, intense weathering leads to the formation of lateritic soils, which are rich in iron and aluminum oxides. These soils form in highly weathered environments, where minerals like feldspar and silicates break down into oxides.
- Example: Bauxite, the primary ore of aluminum, forms in lateritic soils through the weathering of aluminum-bearing silicate rocks. This process involves the removal of silica and other soluble components, leaving behind aluminum oxides, primarily gibbsite.
- Iron Ore: Hematite and goethite, two important iron ores, can also form in lateritic environments through the weathering of iron-rich rocks. The process of supergene oxidation transforms primary iron sulfides or magnetite into these economically significant oxides.
4. Formation of Secondary Enrichment Zones (Supergene Zones)
- Supergene Oxidation: In the supergene zone, which is located near the surface of ore deposits, weathering causes sulfide minerals to oxidize and form secondary oxide minerals. This is a process where metals like copper, iron, and gold are concentrated as oxide minerals due to weathering and subsequent dissolution and reprecipitation.
- Example: Copper deposits in arid regions undergo supergene oxidation, where copper sulfides like chalcopyrite are weathered and transformed into copper oxides like malachite and azurite, which are more easily processed.
5. Transport and Deposition of Oxides
- Transport of Oxides by Water: Weathering can also result in the transportation of oxide minerals through water (rivers, streams, or groundwater), leading to their deposition in secondary environments like alluvial or placer deposits. These transported oxide minerals can accumulate in riverbeds, beaches, or deltas, making them easier to mine.
- Example: Gold oxides or iron oxides can accumulate in alluvial deposits after being weathered and transported from their source rocks, concentrating them in a manner that makes them more accessible and economical to mine.
6. Weathering of Mafic and Ultramafic Rocks (Nickel and Manganese)
- Nickel and Manganese Oxides: Weathering of mafic (magnesium- and iron-rich) and ultramafic rocks (e.g., serpentine) can lead to the formation of nickel and manganese oxide deposits. The weathering process helps concentrate these metals in their oxide forms, making them suitable for mining.
- Example: Nickel laterites form from the weathering of ultramafic rocks, concentrating nickel in the form of garnierite (a nickel silicate), which can later be processed into nickel oxides.
7. Weathering-Induced Ore Enrichment
- Ore Enrichment: Over time, weathering can enhance the concentration of valuable metals in oxide minerals. As the weathering process removes less stable minerals and elements, it can leave behind enriched deposits of more stable oxide minerals.
- Example: In some gold-bearing deposits, weathering concentrates gold in its native oxide form, making it easier to mine and process compared to the original sulfide ores.
Managing the Environmental Impact of Weathering in Mining
While weathering naturally contributes to the formation of valuable oxide mineral deposits, mining activities related to weathered deposits can have significant environmental impacts. To manage these impacts:
- Erosion Control: Implementing effective erosion control measures during mining operations, especially when working in lateritic or tropical environments, can help prevent soil loss and sedimentation in nearby water bodies.
- Water Management: Proper management of water resources and treatment of acid mine drainage (AMD) is essential to prevent contamination from weathered sulfide ores.
- Reclamation and Restoration: Rehabilitating weathered mining sites by restoring native vegetation and improving soil quality is important for ensuring the long-term stability of mined areas.
Conclusion
Weathering plays a fundamental role in the formation of economically significant oxide mineral deposits by transforming primary minerals into more stable oxide forms, often concentrating valuable metals in the process. The formation of oxide deposits through weathering enhances the availability of minerals like bauxite, hematite, manganese oxides, and copper oxides, which are essential to industries such as aluminum, steel, and electronics. By understanding and managing the effects of weathering, the mining industry can access these valuable resources in a more sustainable and environmentally responsible way.