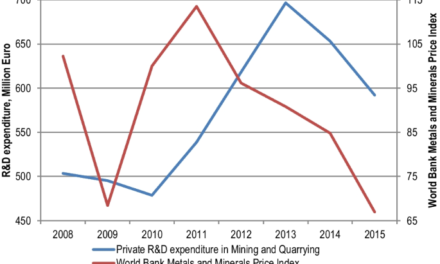
Sulfate minerals are integral to a wide range of industrial applications, including construction, agriculture, and chemical production. However, their beneficiation and refining can be challenging due to their chemical composition and physical properties. Below is a detailed exploration of the most effective techniques used to process sulfate minerals like gypsum, barite, anhydrite, and others.
1. Beneficiation of Sulfate Minerals
Beneficiation refers to the process of concentrating or improving the quality of sulfate minerals by removing impurities. This is typically achieved through methods like gravity separation, flotation, and leaching.
(a) Gravity Separation
- Principle:
- Gravity separation utilizes the density differences between the target mineral and its gangue (waste) materials. This is particularly effective for gypsum, barite, and anhydrite, which have a higher density compared to many gangue minerals.
- Process:
- Crushed ore is subjected to water or air-based separation techniques.
- Jigging, shaking tables, spiral concentrators, and hydrocyclones are common devices used to concentrate minerals based on specific gravity.
- Applications:
- Barite beneficiation—heavy media separation or jigging methods to upgrade barite concentrates.
- Gypsum—separation of coarse and fine fractions, with finer materials being processed separately.
- Advantages:
- Simple, cost-effective, and requires low energy input.
- Environmentally friendly, using only water and gravity.
(b) Flotation
- Principle:
- Flotation separates hydrophobic minerals (minerals that repel water) from hydrophilic minerals (minerals that attract water). This method is typically used for minerals with fine particles or complex associations of different sulfate minerals.
- Process:
- The ore is mixed with water and flotation reagents (collectors, frothers, and modifiers).
- The desired minerals adhere to air bubbles and float to the surface, while gangue minerals sink.
- Applications:
- Barite—can be processed using cationic flotation, where barite is separated from silicate gangue minerals.
- Gypsum—flotation methods are used to remove carbonates and other gangue materials.
- Anhydrite—separation from other sulfate-bearing minerals in complex ores.
- Advantages:
- Effective for fine-grained ores and ores with complex mineralogy.
- Can produce high-purity concentrates.
(c) Leaching and Solution Processes
- Principle:
- Leaching involves using a liquid solvent to selectively dissolve the desired mineral or its components. It is commonly used for gypsum and barite when mineral liberation is not easily achieved through physical separation.
- Process:
- Sulfuric acid leaching is widely used to extract sulfate minerals like gypsum from ores. The mineral reacts with the acid, forming soluble compounds that can be filtered out.
- For barite, leaching can be used to remove iron and other impurities by treating with hydrochloric acid.
- Applications:
- Barite—acid leaching can be employed to remove impurities such as iron oxide.
- Gypsum—the acid-catalyzed process helps purify and concentrate the mineral for various industrial uses.
- Advantages:
- Can be highly selective in dissolving specific impurities.
- Offers high recovery rates for fine-grained minerals.
2. Refining of Sulfate Minerals
Refining refers to processes used to purify and concentrate the target sulfate mineral after beneficiation. Refining typically involves processes like calcination, flotation, and chemical treatments to remove impurities.
(a) Calcination (Thermal Refining)
- Principle:
- Calcination involves heating sulfate minerals to a high temperature to dehydrate or chemically transform them into a more usable form. This method is widely used in refining gypsum and anhydrite.
- Process:
- For gypsum (CaSO₄·2H₂O), heating the mineral at 400–600°C removes the water molecules, transforming it into anhydrite (CaSO₄).
- In some cases, calcined gypsum is then rehydrated to produce high-quality plaster or wallboard products.
- Applications:
- Gypsum—calcination helps in producing plaster of Paris and gypsum board.
- Anhydrite—calcination and sintering processes help convert anhydrite into anhydrous calcium sulfate used in the construction industry.
- Advantages:
- Produces high-quality products for construction and agricultural uses.
- Energy-intensive, but necessary for producing specific mineral forms.
(b) Chemical Refining (Purification via Chemical Reactions)
- Principle:
- Chemical refining processes involve using reagents to selectively remove impurities from sulfate minerals, leaving behind a purified product.
- Process:
- Barite Refining: Barite often contains iron oxide impurities. The ore is treated with hydrochloric acid to remove iron as iron chloride (FeCl₃), leaving purified barite.
- Gypsum Refining: Gypsum may contain organic impurities. It can be treated with sodium carbonate or magnesium compounds to purify and remove contaminants.
- Applications:
- Barite—acid refining can purify barite for use in drilling mud and chemical industries.
- Gypsum—purified gypsum is used for plaster and fertilizer production.
- Advantages:
- High purity can be achieved for industrial applications.
- Chemical methods are selective and versatile, catering to different impurities.
3. Advanced Techniques for Processing Sulfate Minerals
(a) Bioleaching and Bioremediation
- Principle:
- Bioleaching uses microorganisms to extract metals or refine minerals from ores, leveraging the natural biological activity of bacteria, fungi, or algae. This technique has gained attention in the mining industry as a more sustainable and eco-friendly method.
- Process:
- For sulfate minerals, sulfate-reducing bacteria (SRB) can be used to reduce sulfate ions into sulfide minerals. This can help in recovering valuable metals like copper, nickel, and gold.
- The process can also be used for the remediation of AMD by biologically neutralizing acidic waters and precipitating heavy metals.
- Applications:
- Copper and gold recovery from sulfide-rich ores using bioleaching.
- Environmental remediation of mining sites impacted by sulfate-rich wastewaters.
- Advantages:
- Eco-friendly, uses natural biological processes.
- Lower energy consumption compared to conventional methods.
Conclusion
The beneficiation and refining of sulfate minerals—such as gypsum, barite, and anhydrite—involve a combination of physical separation, chemical treatment, and thermal processing techniques. Gravity separation and flotation are the primary methods for mineral concentration, while calcination and chemical refining are used to purify and refine these minerals for industrial applications. Emerging methods like bioleaching are being explored for their sustainability, and with advances in technology, the processing of sulfate minerals will likely become more cost-effective, efficient, and environmentally friendly.
Hashtags
#SulfateMinerals #MineralBeneficiation #OreRefining #SulfateOreProcessing #MineralProcessing #SulfateExtraction #FlotationTechniques #Hydrometallurgy #BeneficiationTechniques #RefiningProcesses #SulfateOreBeneficiation #MiningTechnology #OrePurification #EfficientRefining #MiningInnovation #SulfateMineralProcessing #MineralExtraction #SulfateLeaching #MetallurgicalProcessing #MineralSeparation #SustainableMining