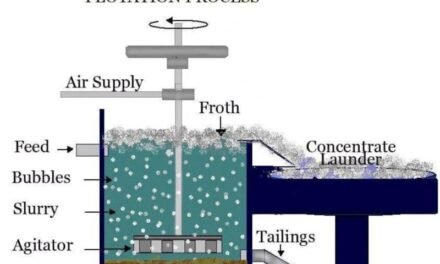
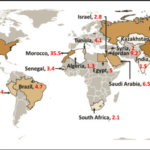
Sulfide minerals are often associated with precious metals like gold, silver, and platinum due to their geological formation processes and the chemical affinity between sulfur and these metals. Here’s why this association exists:
1. Geological Formation
- Sulfide Ores as Host Rocks: Many precious metals, such as gold, silver, and platinum, are commonly found in sulfide-rich mineral deposits. Sulfide minerals, including pyrite (FeS₂), chalcopyrite (CuFeS₂), and galena (PbS), can act as host rocks or carriers for these precious metals. Precious metals often form in the same geological settings, such as hydrothermal veins or volcanic and sedimentary deposits, where sulfur is prevalent.
- Volcanic and Magmatic Processes: In some cases, precious metals form from magmatic processes where sulfur, along with metals, crystallizes from molten rock. The metals precipitate alongside sulfide minerals during cooling and solidification, often within the same vein or ore body.
2. Chemical Affinity
- Sulfur’s Role in Precipitation: Sulfur reacts with metals like gold, silver, and platinum to form sulfide complexes under certain geological conditions. For example, gold often occurs in the form of gold-bearing sulfides such as auriferous pyrite. Similarly, silver may occur in minerals like argentite (Ag₂S) or silver sulfides, and platinum forms platinum-group sulfides.
- Stabilizing Effect of Sulfur: Sulfur helps stabilize these precious metals in low-oxygen, high-sulfur environments, preventing them from oxidizing or reacting with other substances. This is particularly true in deeper or more reducing environments where oxygen is scarce, allowing these metals to remain in sulfide form rather than oxidizing into less stable compounds.
3. Hydrothermal Activity
- Gold and Sulfide Deposition: During hydrothermal activity, hot, sulfur-rich fluids from deep within the Earth travel through rock layers, carrying with them dissolved precious metals. As the fluid cools or encounters changes in pressure, gold and other precious metals precipitate out of the solution, often forming sulfide minerals.
- Platinum-Group Elements (PGEs): Similarly, platinum and other platinum-group elements (PGEs) are commonly found in sulfide ores in regions with past or active magmatic and hydrothermal systems.
4. Concentration in Ore Bodies
- Enrichment by Sulfide Minerals: In many mining districts, sulfide ores act as natural concentrators of precious metals. The geochemical processes that lead to the formation of sulfide ore bodies often concentrate gold, silver, and platinum in specific areas of the ore deposit, making them economically viable to mine.
Conclusion
Sulfide minerals are often associated with precious metals like gold, silver, and platinum because of their geological formation and the chemical processes that occur during the deposition and cooling of mineral-rich fluids. Sulfur plays a crucial role in stabilizing these metals in sulfide form, particularly in hydrothermal environments, making sulfide deposits important sources for precious metals. The interaction between sulfur and metals in geological systems leads to the formation of rich ore bodies where these precious metals are concentrated, facilitating their extraction and industrial use.