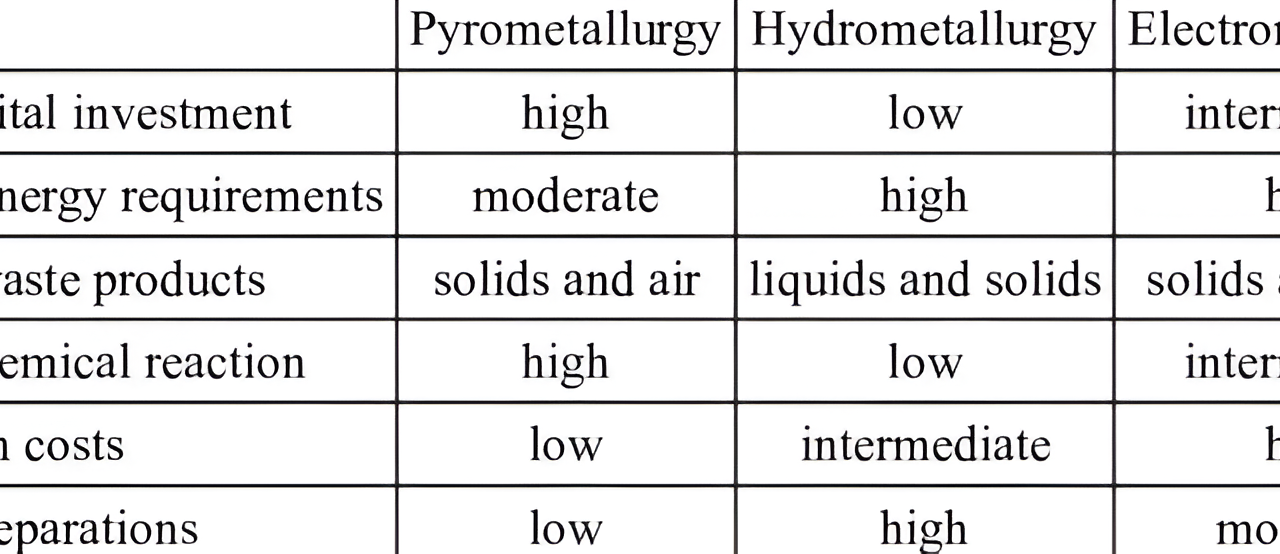
Pyrometallurgy, hydrometallurgy, and electrometallurgy are the three primary methods used in the refining of metals, each utilizing different techniques and principles to extract metals from ores or concentrates. Here’s a breakdown of the differences between these three refining processes:
1. Pyrometallurgy
Pyrometallurgy involves the use of high temperatures to extract and refine metals. It is typically used for ores that contain metals in solid or liquid form, and it relies on heat and chemical reactions to separate the metal from the ore.
Key Features:
- Temperature: High temperatures (often above 900°C or 1,000°C) are used to process the material.
- Process: Pyrometallurgical processes typically involve smelting (melting the ore to separate metals from impurities), roasting (heating the ore in the presence of oxygen to convert sulfides to oxides), and refining (removing impurities from metals).
- Applications: It is commonly used for base metals (e.g., copper, iron, nickel, lead) and some precious metals (e.g., gold, silver).
- Reaction Types: The process involves oxidation, reduction, and fusion reactions to separate the metal from its ore.
- Examples:
- Blast Furnace: Used in iron and steel production.
- Copper Smelting: Copper ores are heated in furnaces to separate copper from impurities.
- Gold Refining: Gold ores are treated with cyanide or other chemicals to separate gold.
Advantages:
- High throughput and scalability.
- Well-established for many metals, especially for extraction from sulfide ores.
Disadvantages:
- Energy-intensive due to the high temperatures required.
- Potential for environmental pollution (e.g., CO₂ emissions, sulfur dioxide from roasting).
2. Hydrometallurgy
Hydrometallurgy involves the use of aqueous solutions (water and chemicals) to extract metals from ores. This process is often employed for metals that are more easily dissolved in water, especially when ores are not amenable to pyrometallurgical methods.
Key Features:
- Solvents: Solvents such as acidic solutions (e.g., sulfuric acid, hydrochloric acid) or alkaline solutions (e.g., sodium cyanide for gold extraction) are used to dissolve the metal.
- Process: The ore is treated with a solution that leaches the metal into solution, and the metal is then extracted from the solution using various methods such as precipitation, solvent extraction, or electrowinning.
- Applications: Hydrometallurgy is commonly used for precious metals (gold, silver), rare earth elements, copper, and other non-ferrous metals.
- Examples:
- Heap Leaching: Used for gold and copper ores, where the ore is piled in heaps and leached with cyanide or sulfuric acid.
- Copper Extraction: Copper is extracted using sulfuric acid to dissolve the metal from copper ores, followed by solvent extraction and electrowinning.
Advantages:
- Lower temperatures compared to pyrometallurgy.
- Less energy-intensive, especially for low-grade ores.
- Can be more selective for certain metals (e.g., gold and copper).
Disadvantages:
- Requires large volumes of chemicals and water.
- Chemical waste disposal and environmental concerns related to leaching agents (e.g., cyanide, acids).
- Slower processing time compared to pyrometallurgy for some metals.
3. Electrometallurgy
Electrometallurgy is a method of refining and extracting metals using electrical energy to drive the reduction or deposition of metals from their ores or concentrates. This process is often used when the metal exists in ionic form and can be reduced by electrolysis.
Key Features:
- Electrical Current: Uses electrochemical processes, where an electric current is passed through a solution containing metal ions or a molten metal to extract the metal.
- Process: The metal is deposited at the cathode of an electrolytic cell, where the metal ions are reduced to solid metal. The process involves electrolysis, where a direct current (DC) is applied to an electrolyte solution or molten salt.
- Applications: Electrometallurgy is commonly used for precious metals (gold, silver), aluminum, copper, and zinc.
- Examples:
- Electrorefining of Copper: Copper is extracted from a copper-rich concentrate through electrolysis, where pure copper is deposited at the cathode.
- Electrowinning: Used for metals like copper, zinc, and gold, where metal ions are reduced from the solution and deposited as solid metal.
- Aluminum Production: The Hall-Héroult process uses electrolysis to extract aluminum from its ore, bauxite.
Advantages:
- High purity of the extracted metal, as electrolysis can produce very high-quality refined metals.
- Lower environmental impact compared to pyrometallurgy, as it doesn’t involve high temperatures.
- Selective extraction can be achieved for specific metals, and it is especially useful for high-value metals like gold and silver.
Disadvantages:
- Requires large amounts of electrical energy, making it energy-intensive.
- Limited applicability: Electrorefining is not suitable for all types of ores or metals, as the metal needs to be in a soluble ionic form.
- Capital-intensive setup and maintenance for electrolysis cells and power supply systems.